
NMPA Down-Classified Delivery Guide Devices and Certain Inner Ear Prostheses
The NMPA issued the “Announcement on Adjustments to Certain Items in the Medical Device Classification Catalog” on January 4, 2026. The adjustments are aimed to
Register for Upcoming Webinar on DEC. 8 @ 11AM
2025 China NMPA Bluebook is here:

The NMPA issued the “Announcement on Adjustments to Certain Items in the Medical Device Classification Catalog” on January 4, 2026. The adjustments are aimed to

NMPA issued the “2026 Medical Device Industry Standards Revisions Plan” today on January 28, 2026. One mandatory and seventy-nine recommended standards will be revised or

In the review reports released in December 2025, eight out of ten pertained to IVD reagents, reflecting the continued advancement and regulatory scrutiny in fields

Here’s the latest China NMPA regulatory and clinical affairs news for medical device and IVDs pros in January 2026. These updates are presented by China

OCT-Guided Ocular Injections Intravitreal injections are a cornerstone of modern retinal therapy, used to deliver anti-VEGF agents for AMD, diabetic macular edema, and other retinal

Optical Coherence Tomography (OCT) has become an indispensable imaging tool in ophthalmology, offering high-resolution, non-invasive, real-time cross-sectional visualization of ocular tissues. Since its introduction in

Accurate medical device classification is the foundation of regulatory compliance, registration strategy, clinical planning, and market access in China. NMPA Classification Catalog was published in

Starting January 1, 2026, NMPA will further expand its pre-submission technical consultation program for medical device registration. In addition to the National Center, three regional
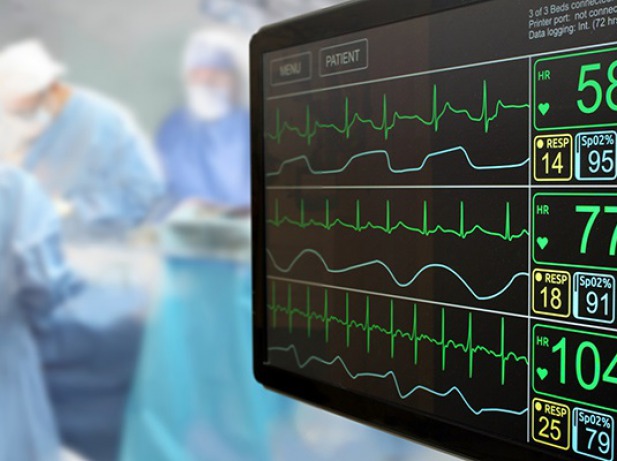
NMPA published 39 medical devices final guidelines on December 1, 2025. They are aimed to facilitate manufacturers with clarity in registration process. Significance of Device
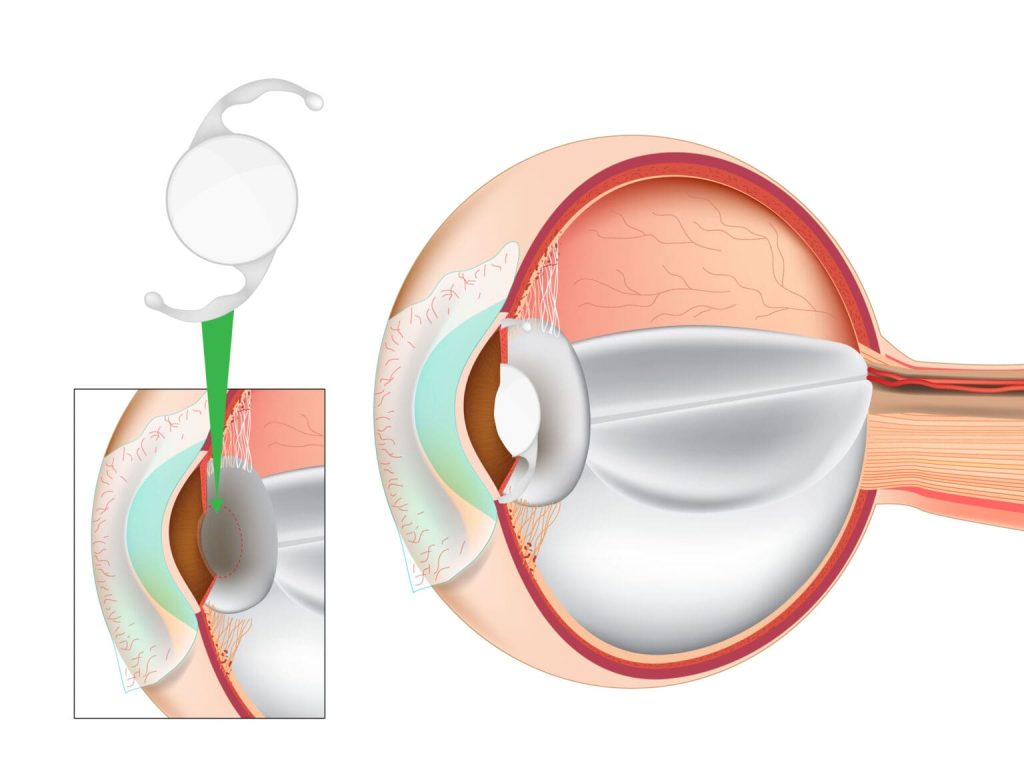
Cataract remains one of the most common ophthalmic diseases in China, largely driven by the country’s rapid demographic transition toward an aging society. Epidemiological reports

Here’s the latest China regulatory and clinical affairs news for medical device and IVDs pros. These updates are presented by China Med Device, LLC, your
The China State Council released the “Administrative Regulation on Clinical Research and Clinical Transformation of New Biomedical Technologies” on October 10, 2025, which will be