
NMPA (CFDA) News Roundup 201910
The NMPA (CFDA) News Roundup covers government announcements, policies, standards, guidelines, QA/recall/AE, and new approvals in medical devices and IVDs in China. It is edited by China Med Device, LLC
Register for Upcoming Webinar on DEC. 8 @ 11AM
2025 China NMPA Bluebook is here:

The NMPA (CFDA) News Roundup covers government announcements, policies, standards, guidelines, QA/recall/AE, and new approvals in medical devices and IVDs in China. It is edited by China Med Device, LLC

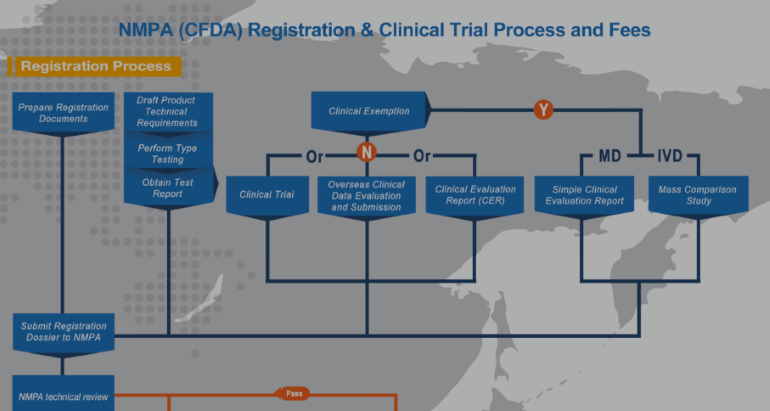
Time: Tuesday, November 12, 2019 | 11:00 am – 12:00 pm ET Presented by China Med Device, LLC Register Here This webinar will cover different clinical pathways and how to
China National Medical Products Administration regulates medical devices and pharmaceutical products across China. The NMPA was also known as China Food and Drug Administration in the past. For medical device

NMPA issued 28 draft guidelines on October 18 for feedback. The guidelines cover one class III device, 27 class II devices and IVDs (cardiovascular, gastroenterological, urological, dental, gynecological and pediatric

NMPA published the final version of Unique Device Identification (UDI) Rules on August 27, 2019, with implementation date of October 1, 2019. The Rules are to adequately identify medical devices

Leaders discussed the Real World Data (RWD) Pilot Program — a cost-effective and less time-consuming alternative to the traditional clinical trial — at a meeting in Boao, Hainan Province. China’s

In this article we will provide an update on Clinical Evaluation Report (CER) requirements in China by NMPA (CFDA). Furthermore, you will learn about areas that you must pay special

The Monthly NMPA (CFDA) News Roundup covers government announcements, policies, standards, guidelines, QA/recall/AE, and new approvals in medical devices and IVDs in China. It is edited by ChinaMed Device, LLC

International Medical Device Regulators Forum (IMDRF) Committee Members meeting was held on September 17-19, in which China NMPA achieved two milestones. The commitments, on post-market-surveillance (PMS) and clinical evaluation, will

NMPA (CFDA) issued the First Group of Unique Device Identification Devices (Draft) for feedback on September 17, and specified the medical devices going into UDI implementation. Devices Impacted In early

NMPA (CFDA) published the “Guideline on Raw Material Change Evaluation of Non-active Device (Draft)” on June 27. Discussing design control, change control, Basic Safety and Effectiveness List, evaluation pathways, implications

NMPA News Roundup covers government announcements, policies, standards, guidelines, QA/recall/AE, and new approvals in medical devices and IVDs in China. Keep yourself updated with NMPA News Roundup, click HERE to